AI Research Reveals Crucial NOD2–Girdin Partnership Driving Crohn's Disease
- Nov 18, 2025
- 3 min read

For decades, the pathology underlying Crohn's Disease—a severe form of inflammatory bowel disease (IBD) characterized by chronic inflammation in the digestive tract—has remained partially obscured. Scientists have long known that variations in the NOD2 gene are strongly linked to the condition, but the exact mechanism behind its role in triggering inflammation was a lingering mystery. Thanks to a powerful integration of molecular biology and AI research, scientists from the University of California, San Diego (UC San Diego) have finally resolved this 25-year-old debate, revealing a crucial two-protein partnership that, when broken, causes the immune system to spiral out of control.
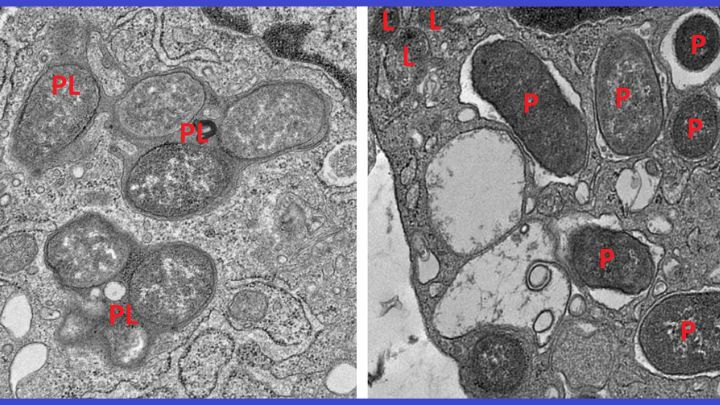
The breakthrough centered on understanding the behavior of specialized immune cells called macrophages, which act as the gut's "peacekeepers". Macrophages must constantly switch between two critical roles: an inflammatory state for attacking infection, and a non-inflammatory state for repairing damage. In Crohn's Disease, this balance collapses, leading to perpetual inflammation and tissue damage.
To decode this imbalance, researchers trained a machine learning algorithm to analyze thousands of macrophage gene expression patterns from both healthy and diseased colon tissue. This AI research identified a genetic signature involving 53 genes that accurately separates inflammatory, reactive macrophages from healing, non-inflammatory ones.
The key discovery involved a protein called girdin (also known as GIV), which was found to promote the non-inflammatory macrophage mode. It turns out that the NOD2 protein functions as the body’s infection surveillance system, but it only maintains gut immune balance effectively when bound to girdin. Without this partnership, the surveillance system "collapses".
The research explains why common mutations in NOD2 are so devastating: they often delete the specific region (the leucine-rich repeat or LRR) where girdin is supposed to attach. This prevents macrophages from shifting from battle mode to healing mode, keeping inflammatory genes active and halting tissue repair.
Experiments on mice confirmed the disastrous consequences of this broken bond. Mice lacking girdin developed severe intestinal inflammation, thickened intestines, and often died of sepsis—a fatal systemic immune overreaction.
These findings offer deep molecular insight into IBD progression and highlight the importance of the NOD2–girdin interaction for intestinal health. Since current Crohn's Disease treatments often broadly suppress the immune system, this targeted understanding paves the way for a wiser approach. Researchers now aim to develop therapies, potentially girdin-mimetic drugs, that can restore this vital partnership, rebalancing macrophages and offering a new path to ease chronic inflammation. The ability of AI research to track these microscopic "players" for the first time marks a significant stride toward personalized treatment.
Analogy: Think of the gut like a town protected by police (macrophages). The NOD2 protein is the police dispatcher, constantly monitoring for threats. The girdin protein is the critical software update that allows the dispatcher to tell the police when to switch from "attack mode" (handling a crime) to "patrol/repair mode" (cleaning up the streets). If the NOD2 gene is mutated, it's like the dispatcher's system lacks the port to install the girdin software. The dispatcher (NOD2) remains active, but it can never signal the police to stand down, leading to an endless, destructive, and exhausting state of siege.
🔖 Sources
Keywords: Crohn's Disease










Four years ago, my husband was diagnosed with IPF (Idiopathic Pulmonary Fibrosis), a moment that changed our lives significantly. For more than two years, he followed his prescribed medications and attended regular medical checkups. Despite this, his symptoms persisted, and we remained concerned about his overall health. He struggled with low energy, frequent discomfort, and the emotional stress that came with ongoing uncertainty.In search of additional support, we decided last year to explore a herbal treatment program offered by NaturePath Herbal Clinic. We approached it cautiously and without high expectations. Over time, however, we began to notice encouraging changes. His tiredness eased, his digestion became more stable, and he appeared stronger and more at ease overall. Little by little, his…